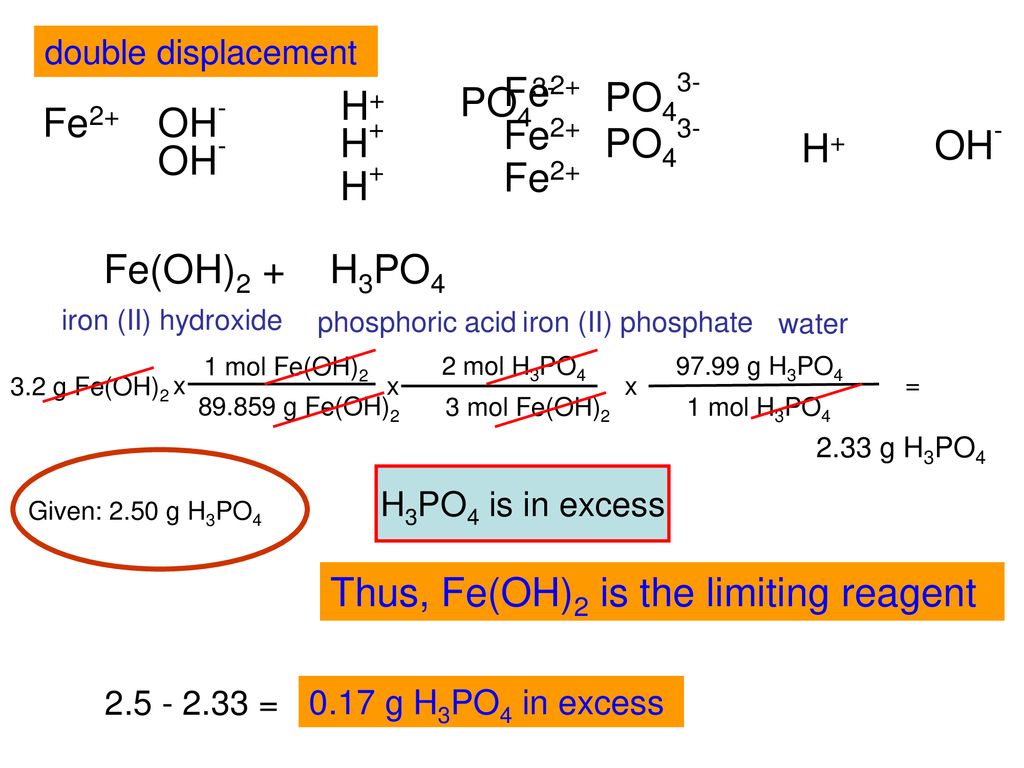
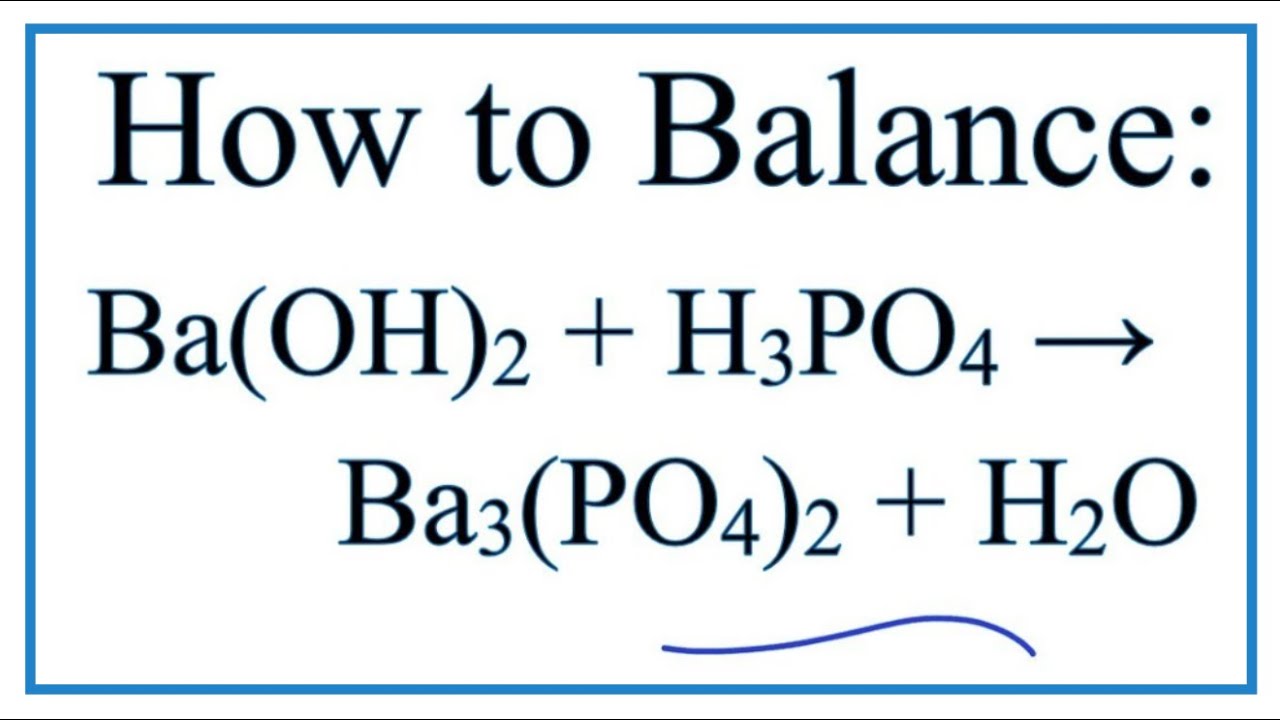
Ca(OH)2 +H3PO4 =Ca3(PO4)2+ H2O Balanced Equation|Cacium Hydroxide +Phosphoric Acid Balanced Equation - YouTube
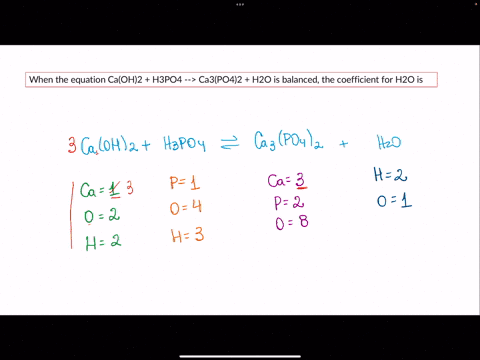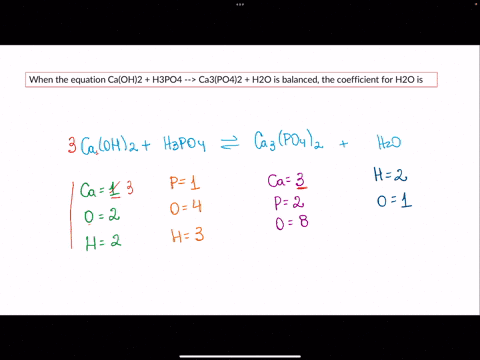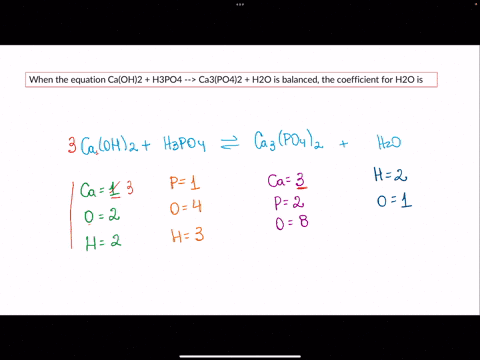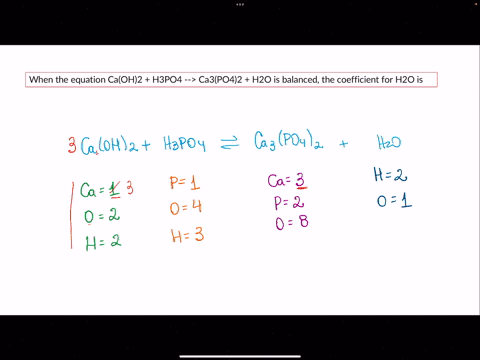
SOLVED: When the equation Ca(OH)2 + H3PO4 –> Ca3(PO4)2 + H2O is balanced, the coefficient for H2O is
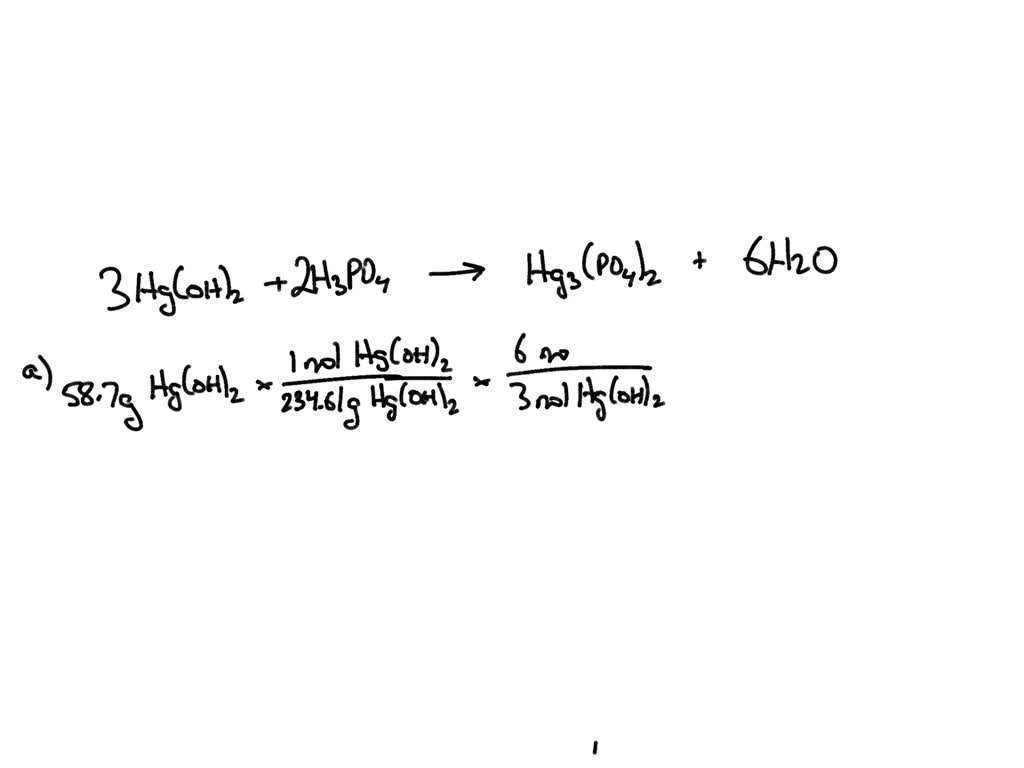
SOLVED: Use the reaction to solve the following problems. Hg(OH)2 + H3PO4→ Hg3(PO4)2 + H2O A) Given 58.7 grams of Hg(OH)2, how many moles of H2O will be produced? If you react

Spex CertiPrep AS-PO49-2Y Phosphate (PO4)-3 Single-Element Ion Anion Standard, 1,000 µg/mL in H2O; 125 mL from Cole-Parmer India
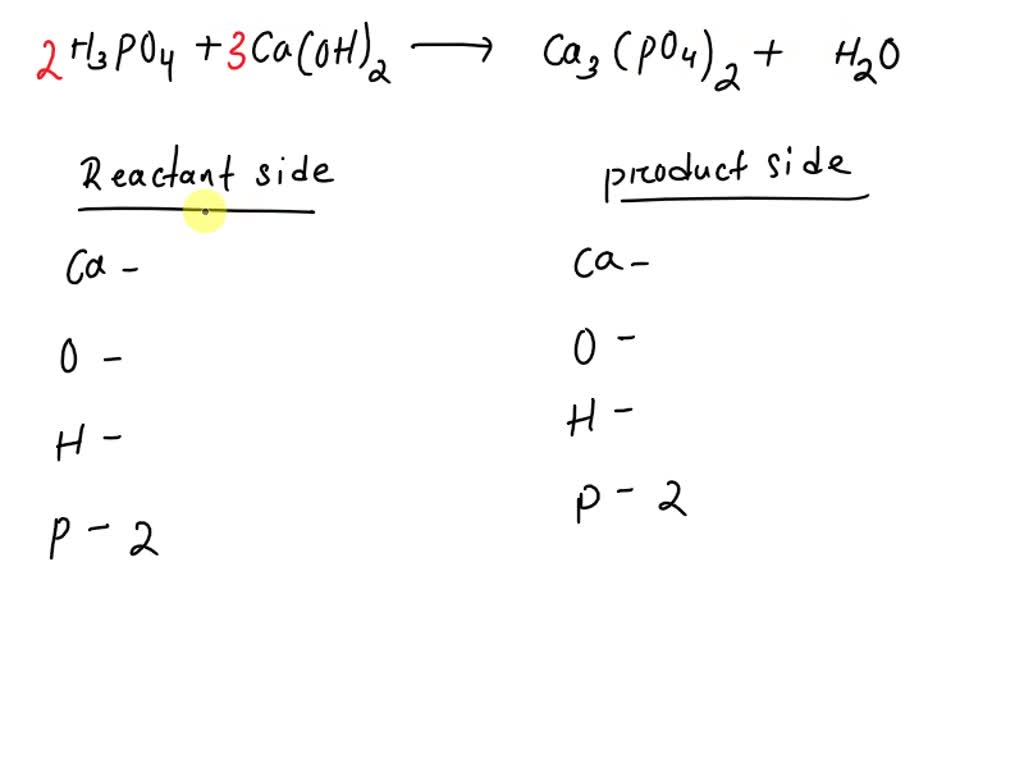
SOLVED: 1. Balance the equation H3PO4 + Ca(OH)2 —> Ca3(PO4)2 + H2O and show the tally table. 2. Draw a colored particle diagram of your total balanced chemical equation. Show a key.

Synthesis and Characterization of a Novel Hydrated Layered Vanadium(III) Phosphate Phase K3V3(PO4)4·H2O: A Functional Cathode Material for Potassium-Ion Batteries | ACS Omega




![Crandallite(Ca[Al3(OH)5(PO4)2].H2O) 1318-36-1 wiki Crandallite(Ca[Al3(OH)5(PO4)2].H2O) 1318-36-1 wiki](https://structimg.guidechem.com/1/1/290580.png)