a) ELF on Se/γ-NiOOH, γ-NiOOH and NiO/α-FeOOH models. (b), (c) The DOS... | Download Scientific Diagram
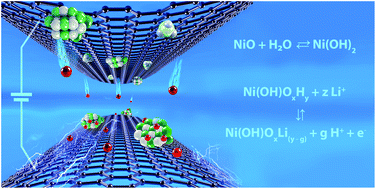
Charge-storage mechanism of highly defective NiO nanostructures on carbon nanofibers in electrochemical supercapacitors - Nanoscale (RSC Publishing)

Kinetic Study of Ni and NiO Reactions Pertinent to the Earth's Upper Atmosphere | The Journal of Physical Chemistry A
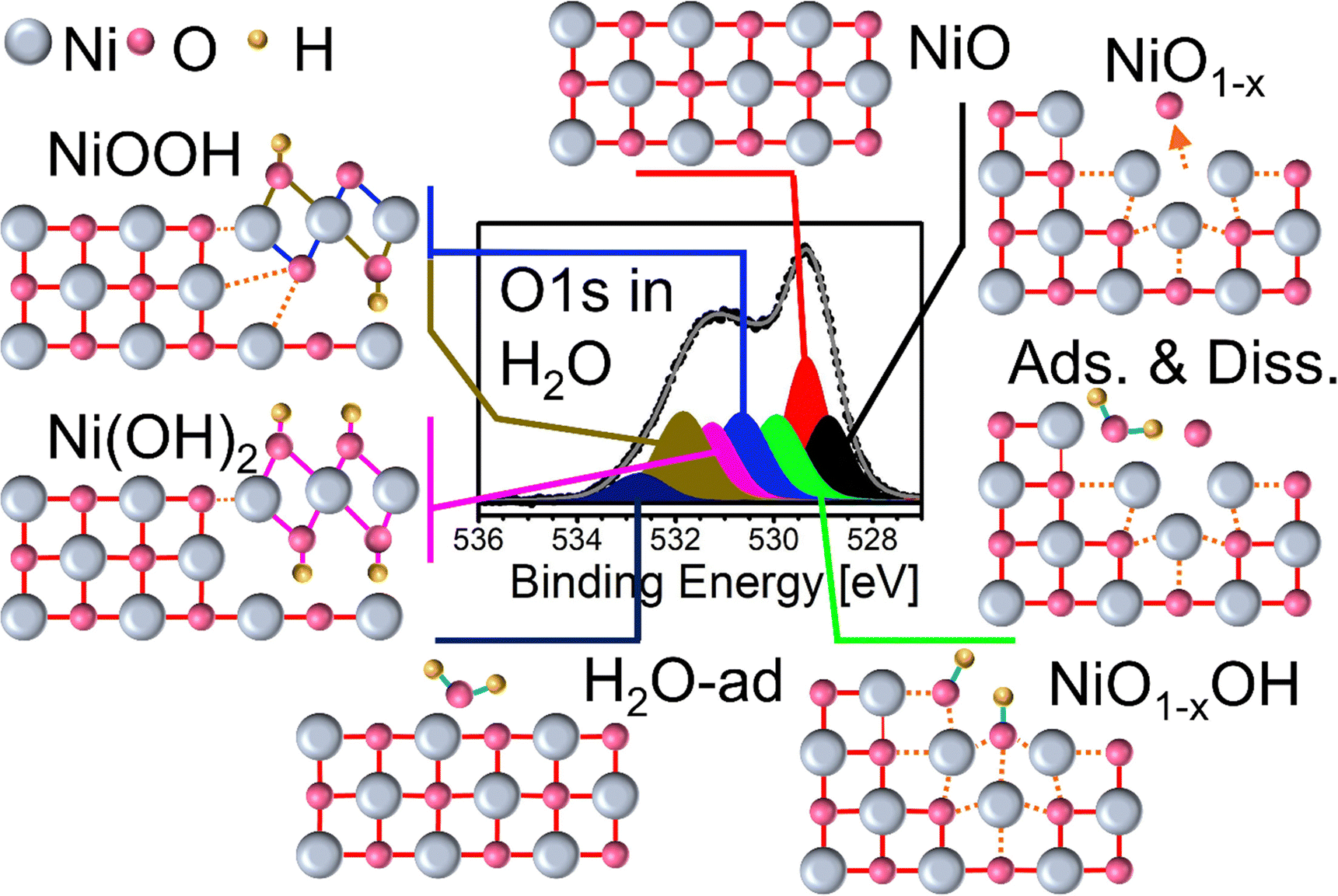
Structural and chemical properties of NiO x thin films: the role of oxygen vacancies in NiOOH formation in a H 2 O atmosphere - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D3CP02047A

The Calotropis procera Transformed Green NiO and Fe‐NiO Nanoparticles for Diaryl Pyrimidinones Synthesis in Hydrotropic Medium at Room Temperature - Shinde - 2018 - ChemistrySelect - Wiley Online Library
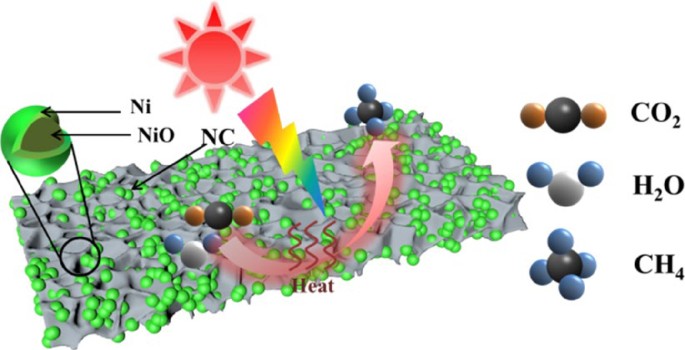
NiO@Ni nanoparticles embedded in N-doped carbon for efficient photothermal CO2 methanation coupled with H2O splitting | Nano Research

Calculated physical parameters such as energy (E) as eV, total dipole... | Download Scientific Diagram

Sm doped NiO catalysts with excellent H2O resistance for N2O decomposition under simulated nitric acid plant exhaust condition - ScienceDirect

Single-Orientation Nanoporous NiO Films: Spontaneous Evolution from Dense Low-Crystalline Ni(OH)x Films | Crystal Growth & Design

Wet etch rate of NiO in 1:4 HNO3:H2O as a function of solution temperature. | Download Scientific Diagram

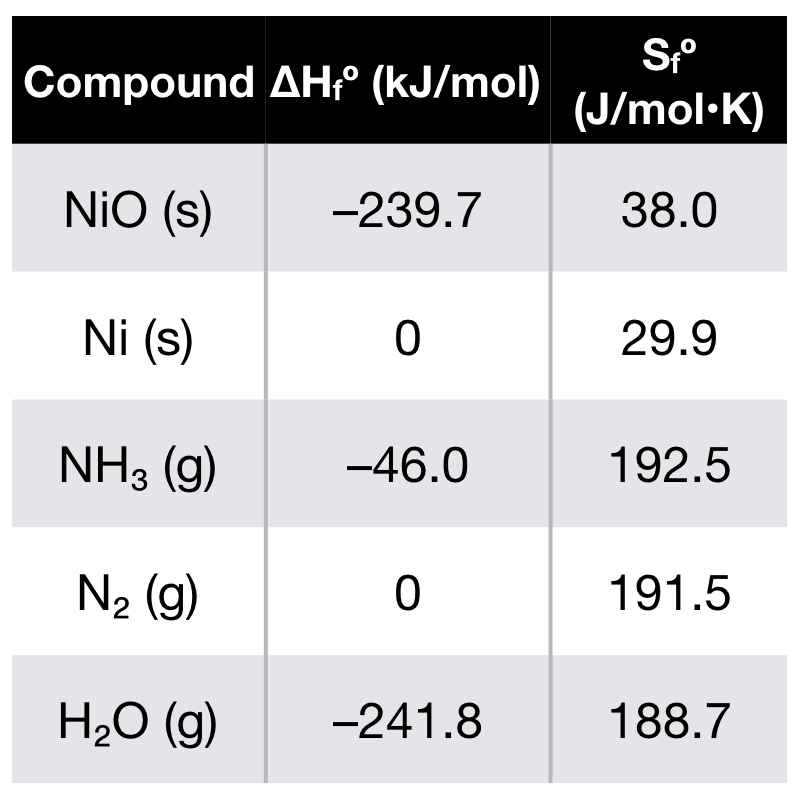
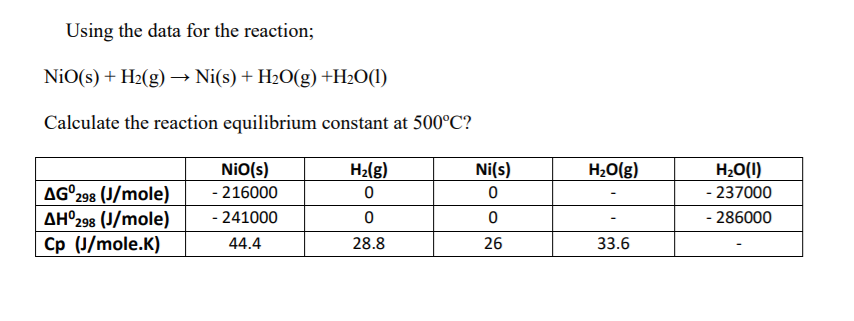
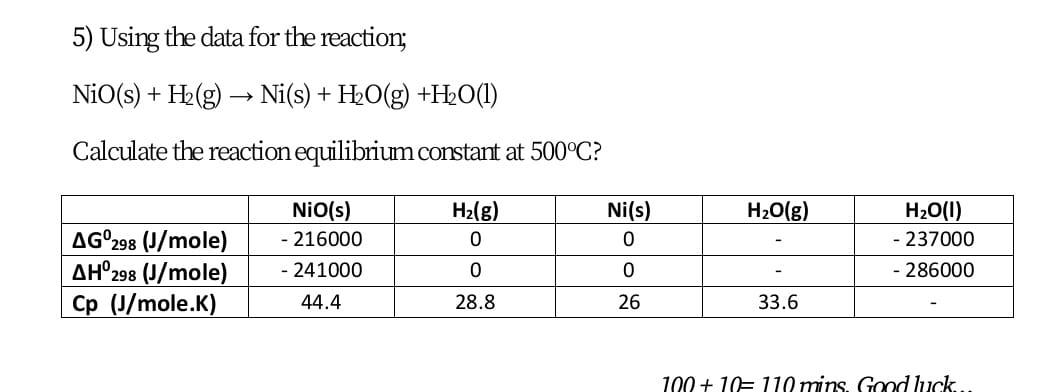
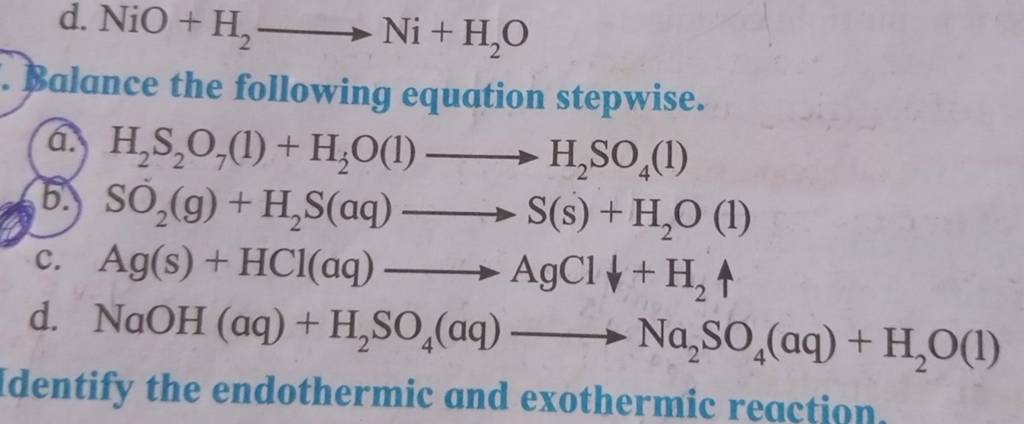
Identify from the following reactions the reactants that undergo oxidation and reduction.NiO+H2⟶Ni+H2O

Kinetic Study of Ni and NiO Reactions Pertinent to the Earth's Upper Atmosphere | The Journal of Physical Chemistry A