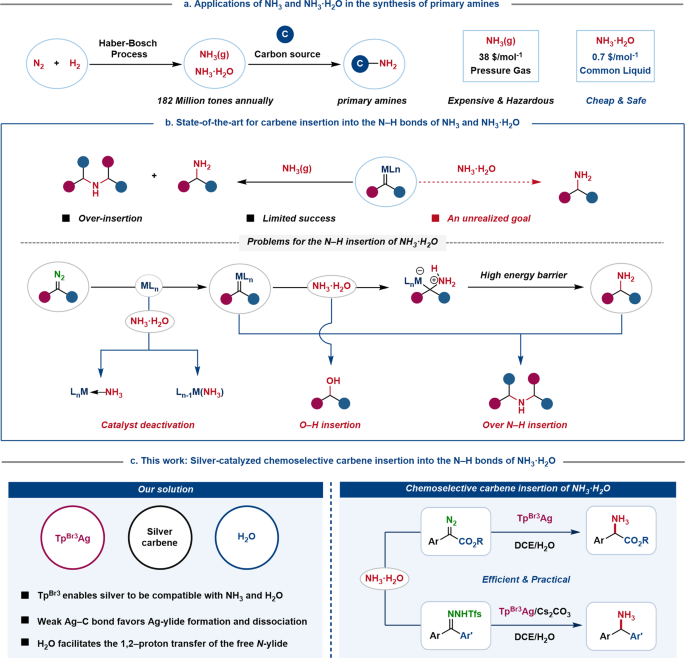
NH3⋅H2O: The Simplest Nitrogen‐Containing Ligand for Selective Aerobic Alcohol Oxidation to Aldehydes or Nitriles in Neat Water - Zhang - 2018 - ChemistryOpen - Wiley Online Library

Effect of water and ammonia on the HO + NH3 → NH2 + H2O reaction in troposphere: Competition between single and double hydrogen atom transfer pathways - ScienceDirect

Experimental Study Of The Binding Energy Of NH3 On Different Types Of Ice And Its Impact On The Snow Line Of NH3 And H2O - Astrobiology

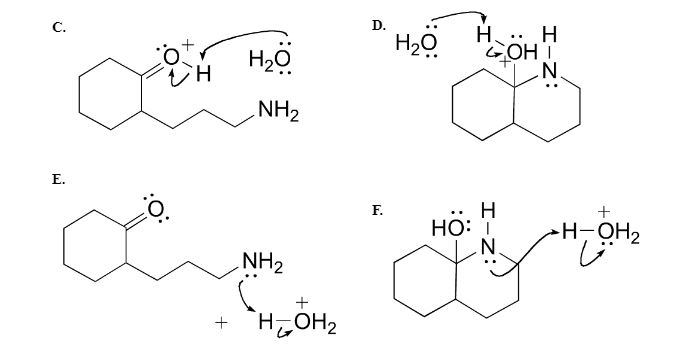
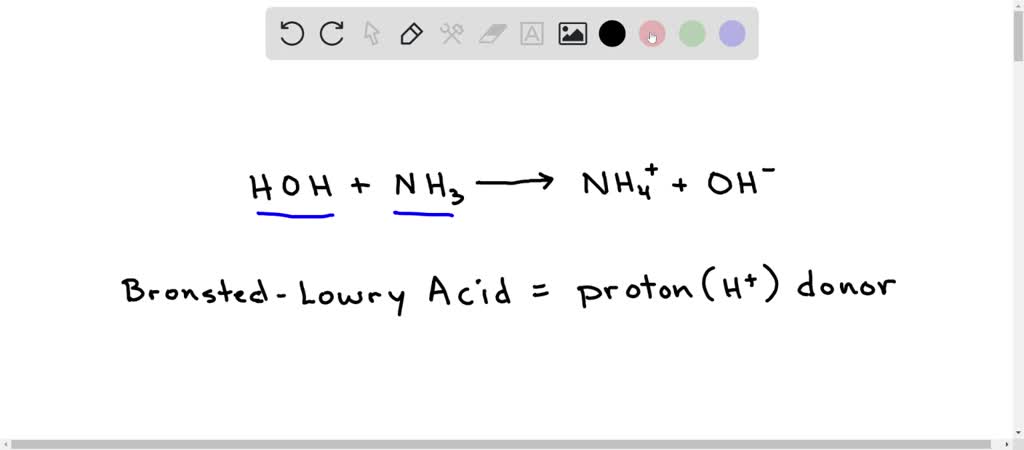
H,0(1) + NH3 (aq)OH (aq) + NH+ (aq) H2O(1)+H,S(aq)| H,O* (aq) +HS- (aq) The auto-protolysis (self-ionization) of water takes place as follows: H2O(1) + H2O(1) H,O* (aq) + OH- (aq) acid-1 base-2
Catalytic effect of (H2O)n (n = 1–3) on the HO2 + NH2 → NH3 + 3O2 reaction under tropospheric conditions - RSC Advances (RSC Publishing)
Although the geometries of NH3 and H2O molecules are distorted, the tetrahedral bond angles in water is less than that of ammonia. Why? - Quora

L'approvisionnement (AMH) de NH3· H2O Prix d'hydroxyde d'ammonium - Chine Hydroxyde d'ammonium, l'Ammoniac L'EAU

Effect of water and ammonia on the HO + NH3 → NH2 + H2O reaction in troposphere: Competition between single and double hydrogen atom transfer pathways - ScienceDirect
The correct option(s) regarding the complex [Co(en) (NH3)3(H2O)]^3+ :- (en = H2NCH2CH2NH2) is (are) - Sarthaks eConnect | Largest Online Education Community






![The CO2-NH3-H2O system as described by the Thomsen model [7]. | Download Scientific Diagram The CO2-NH3-H2O system as described by the Thomsen model [7]. | Download Scientific Diagram](https://www.researchgate.net/publication/319196113/figure/fig1/AS:533643246669824@1504241872081/The-CO2-NH3-H2O-system-as-described-by-the-Thomsen-model-7.png)