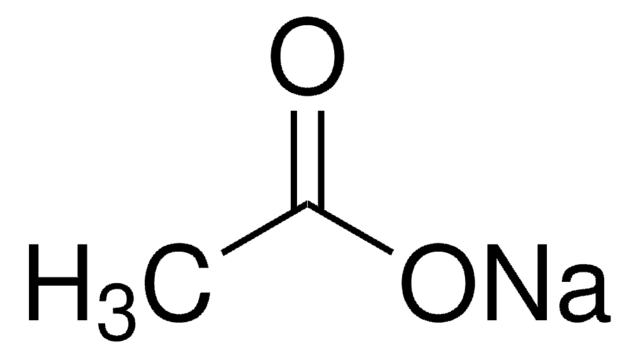
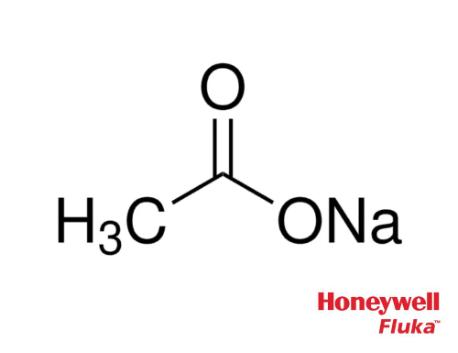
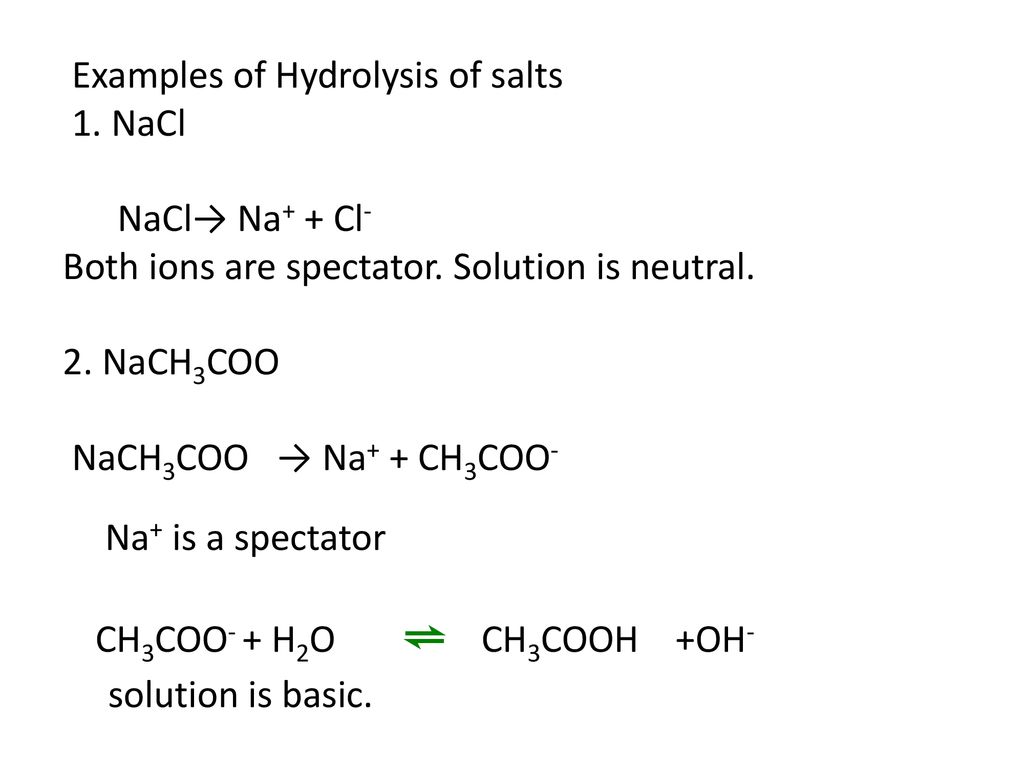
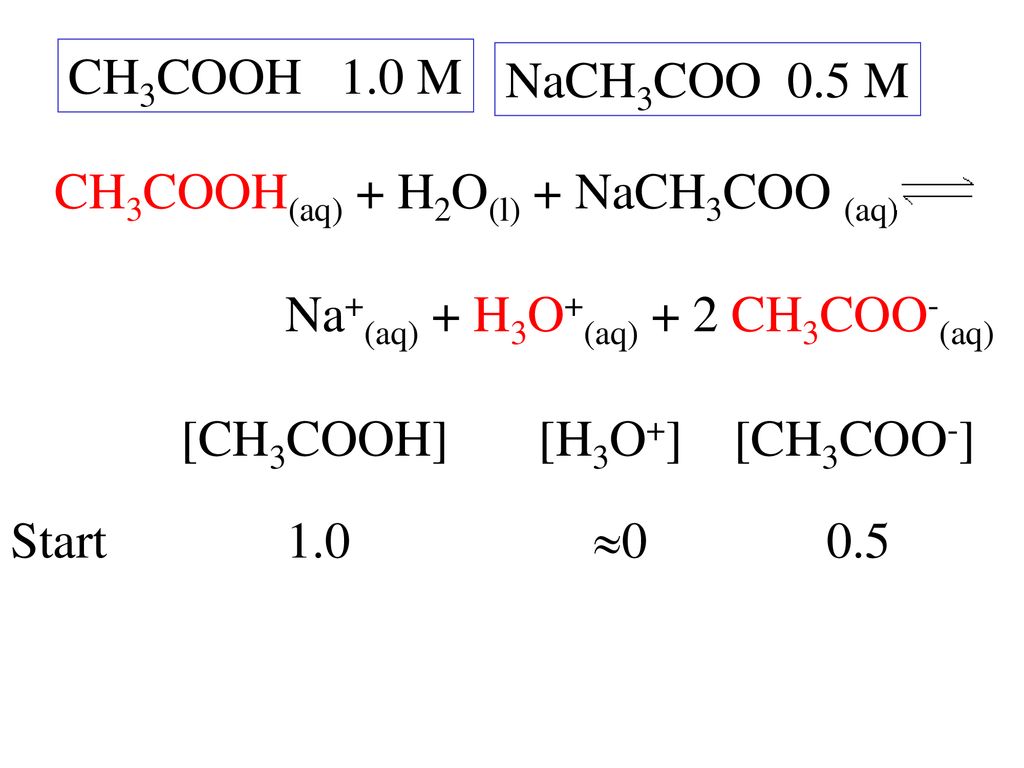
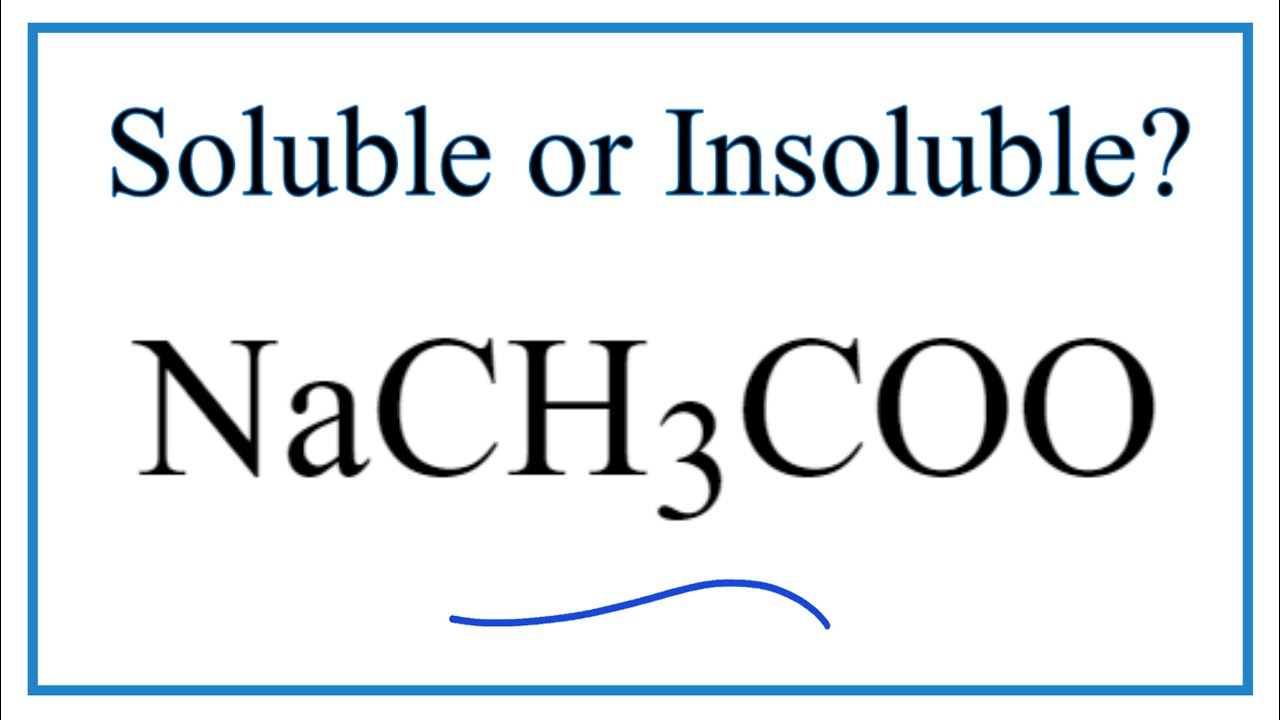For sodium acetate solution in water, the given equilibrium reaction occur:CH 3 COO aq + H 2 O l hydrolysis ⇌ CH 3 COOH aq + OH aqWhich of the following describes

In a reaction 5.3g of sodium carbonate reacted with 6g of ethanoic acid the products were 2.2g of carbon dioxide 0.9g water and 8.2g of sodium ethonoate show that these observations are
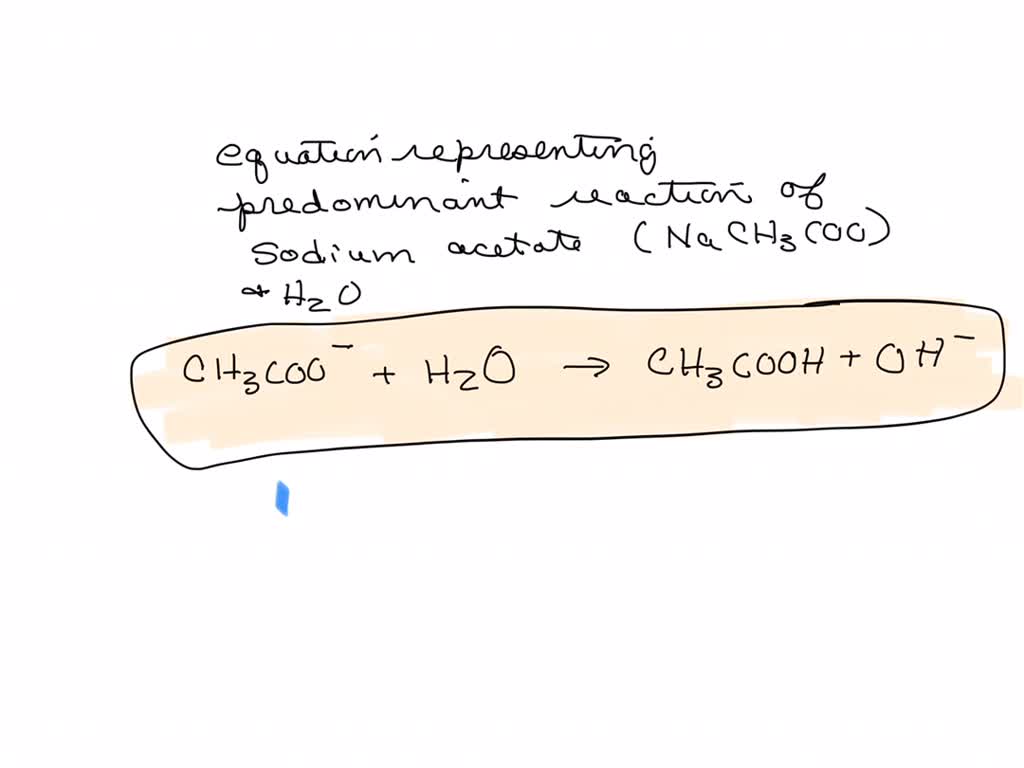
SOLVED: The equation representing the predominant reaction of sodium acetate, NaCH3COO, with water is: CH3COO- + H2O ⇌ CH3COOH + OH- CH3COO- + H2O ⇌ CH3COOH + OH- CH3COOH + H2O ⇌
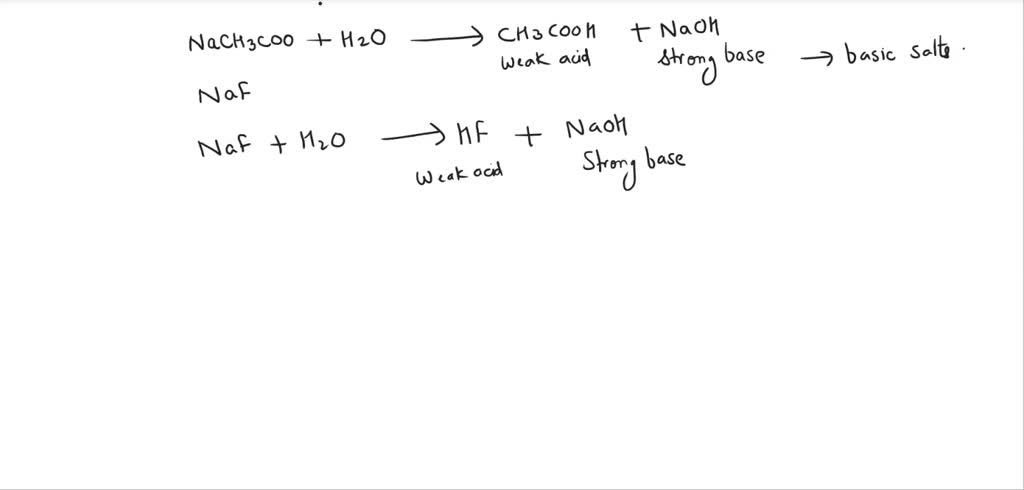
SOLVED: Write chemical equations to show that NaCH3COO and NaF are basic salts. Indicate which of the two salts are more basic in aqueous solutions, and explain why.

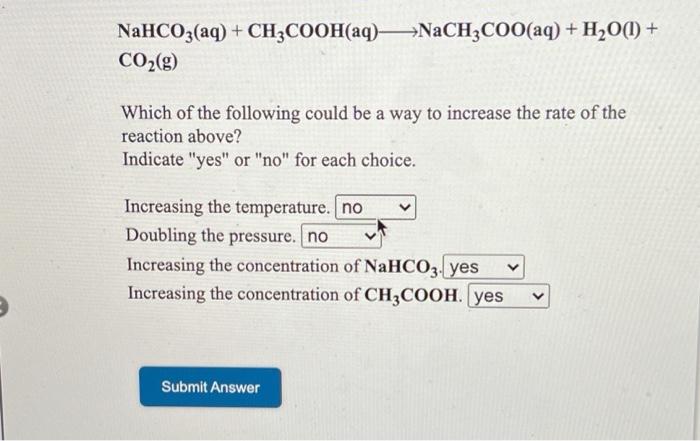
SOLVED: NaHCO3(aq) + CH3COOH(aq) â†' CO2(g) + NaCH3COO(aq) + H2O(l) Which of the following could be a way to increase the rate of the reaction above? Indicate "yes" or "no" for each

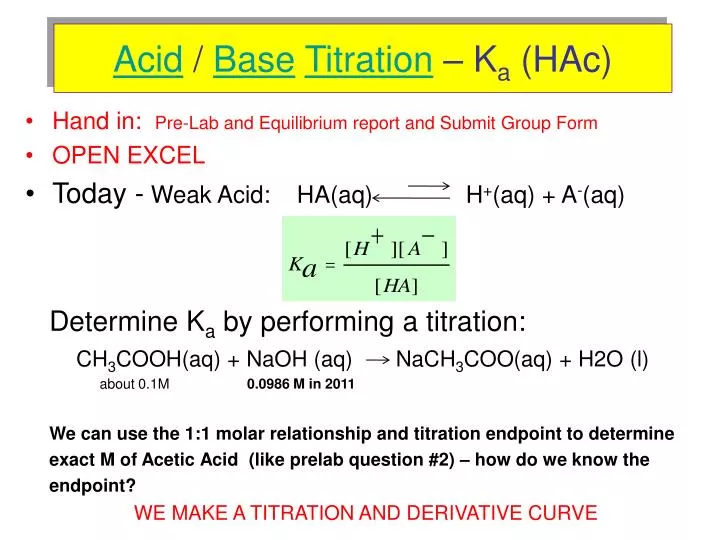
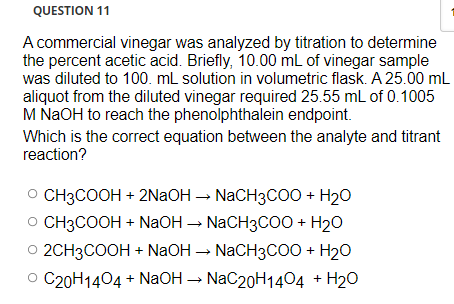
NaOH + X ----> NaCH3COO + H2O What is X in this reaction? A-NH4OH B-H3PO4 C-H2CO3 D-CH3COOH - brainly.com





![Sodium Acetate Trihydrate [CH3COONa.3H2O] Molecular Weight Calculation - Laboratory Notes Sodium Acetate Trihydrate [CH3COONa.3H2O] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2022/12/sodium-acetate-trihydrate-molecular-weight-calculation-300x228.jpg)