SOLVED: QUESTION 11 Is H2O or H2S more nucleophilic in protic solvent, and why? H2S is more nucleophilic because it is less solvated by the protic solvent: 0 H2O is more nucleophilic

Hydrogen Sulfide, Corrosion and BOD5 in Innovyze InfoSewer – ICM SWMM & ICM InfoWorks, SWMM5 & SWMM5+

Hydrate Stability in the H2S–H2O system—Visual Observations and Measurements in a High-Pressure Optical Cell and Thermodynamic Models | Journal of Chemical & Engineering Data

H2O is liquid while H2S is a gas why - Chemistry - The p-Block Elements - 14956293 | Meritnation.com

Comparison between hydrogen production via H2S and H2O splitting on transition metal-doped TiO2 (101) surfaces as potential photoelectrodes - ScienceDirect
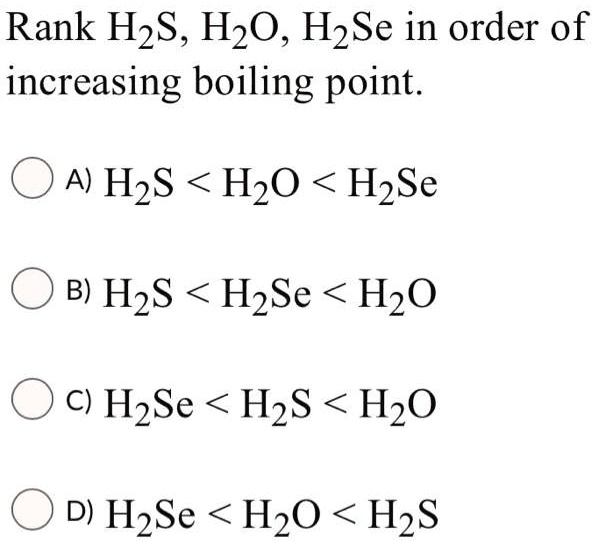



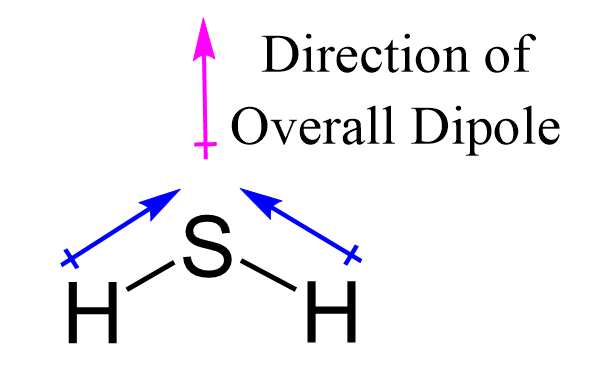








![Punjabi] H2S is a gas while H2O is liquid at room temperature·? Why ? Punjabi] H2S is a gas while H2O is liquid at room temperature·? Why ?](https://static.doubtnut.com/ss/web-overlay-thumb/10548186.webp)