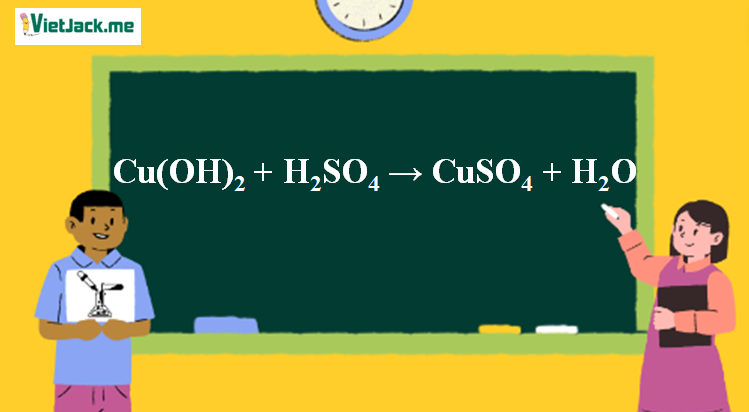Copper hydroxide -Cu(OH)2 - Structure, Molecular Mass, Physical Properties, Chemical Properties, Uses and FAQs of Copper hydroxide.

Fabrication and characterization of Cu(OH) 2 /CuO nanowires as a novel sensitivity enhancer of the luminol–H 2 O 2 chemiluminescence system: determina ... - RSC Advances (RSC Publishing) DOI:10.1039/C5RA21085B
![MaChemGuy ⌬ on X: "[Cu(OH)2(H2O)4] formed by the addition of a small amount of NH3(aq) to [Cu(H2O)6]2+ https://t.co/oDVhJY056M" / X MaChemGuy ⌬ on X: "[Cu(OH)2(H2O)4] formed by the addition of a small amount of NH3(aq) to [Cu(H2O)6]2+ https://t.co/oDVhJY056M" / X](https://pbs.twimg.com/media/DcshHbyX4AAZSXf.jpg:large)
MaChemGuy ⌬ on X: "[Cu(OH)2(H2O)4] formed by the addition of a small amount of NH3(aq) to [Cu(H2O)6]2+ https://t.co/oDVhJY056M" / X

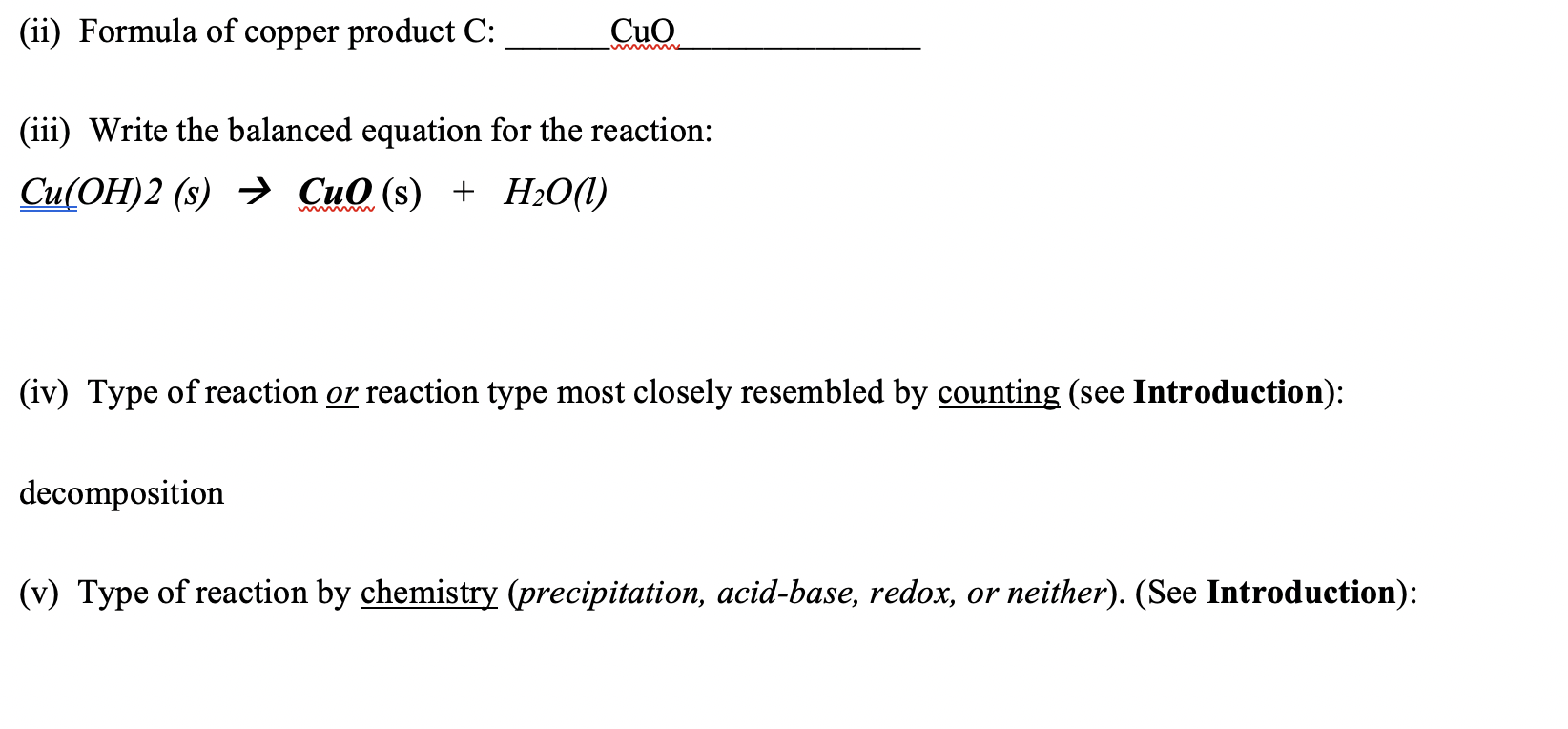
H Balance the following reactions NaOH + H2SO4 → Na2SO4+H2O 7 CuSO4 + NaOH → Cu(OH)2 + Na2SO4 Fe + H2O → Fe3O4+H2 4. NH4Cl + Ca(OH)2 CaCl2 + H2O + NH3 6. Fe + Cl2 → FeCl3 3. Fe

Figure 2 from Simple Template-Free Solution Route for the Controlled Synthesis of Cu(OH)2 and CuO Nanostructures | Semantic Scholar
Crystal structure of Cu(OH) 2 (a) and a proposed structure model of... | Download Scientific Diagram
![SOLVED: BER 2: PRECIPITATION OF Cu(OH)2 WITH NaOH SOLUTION The [Cu(H2O)]2+ is precipitated with NaOH. The OH neutralizes the excess nitric acid and combines with Cu2+ to form insoluble Cu(OH)2. Neutralization reaction: SOLVED: BER 2: PRECIPITATION OF Cu(OH)2 WITH NaOH SOLUTION The [Cu(H2O)]2+ is precipitated with NaOH. The OH neutralizes the excess nitric acid and combines with Cu2+ to form insoluble Cu(OH)2. Neutralization reaction:](https://cdn.numerade.com/ask_images/2689c8e6e30c449396746451a7ccf835.jpg)
SOLVED: BER 2: PRECIPITATION OF Cu(OH)2 WITH NaOH SOLUTION The [Cu(H2O)]2+ is precipitated with NaOH. The OH neutralizes the excess nitric acid and combines with Cu2+ to form insoluble Cu(OH)2. Neutralization reaction:


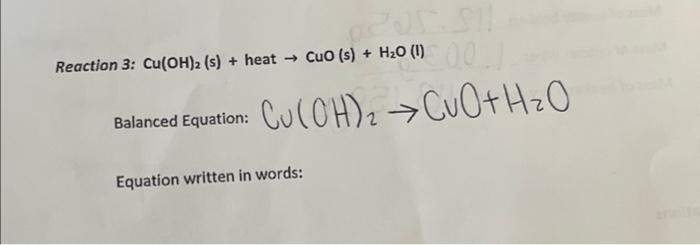






![Cu(OH2)6]2+ - Copper(II) hydroxide Cu(OH2)6]2+ - Copper(II) hydroxide](https://www.chemtube3d.com/images/gallery/inorganicsjpgs/cuoh262_.jpg)