Influence of calcium peroxide on fermentation pattern and protozoa in the rumen: Archiv für Tierernaehrung: Vol 32, No 7-8

Polymers | Free Full-Text | Synthesis of Controlled-Release Calcium Peroxide Nanoparticles Coated with Dextran for Removal of Doxycycline from Aqueous System

C) Cal24 Pulice (d) All of these - Cl2 2 → A - Auto-oxidation Ca(OH)2 -H20 → CaCl2 + B Dry cao2 Identify B in the above reaction :

Please help with all parts and show work. The decomposition of Ca(OH)_2(s) into CaO(s) and H_2O(g) at constant pressure requires the addition of 109 kj of heat per mole of Ca(OH)_2. (a)
Applied Sciences | Free Full-Text | Comparative Kinetic Analysis of CaCO3/CaO Reaction System for Energy Storage and Carbon Capture

Fabricating collagen films with oxygen-release capabilities: 1,7-octadiene PECVD encapsulation of calcium peroxide - American Chemical Society

CaO2/UV Process for One-Step Phosphorus Removal and Recovery from Hypophosphite: Simultaneous Oxidation and Precipitation | ACS ES&T Water

C) Cal24 Pulice (d) All of these - Cl2 2 → A - Auto-oxidation Ca(OH)2 -H20 → CaCl2 + B Dry cao2 Identify B in the above reaction :
Consider the following reaction sequence:12.CaCO3(s)+ 2HCI(aq)CaCl2(aq) + CO2(g) + H20heatCaO(s) + H20(g).CaCO3(s)If the percentage yield of the 1st step is 80

SOLVED: Consider the combustion of Ca. What are the expected products of the reaction? Select all that apply: CaO2 CaZo H2O CaO CO2

Identify the substance oxidized, substance reduced, oxidizing agent and reducing agent in the following: 1 Cl2 + 2NaBr 2NaCl + - Science - Chemical Reactions and Equations - 13638355 | Meritnation.com
Novel iron sand-derived α-Fe2O3/CaO2 bifunctional catalyst for waste cooking oil-based biodiesel production | Environmental Science and Pollution Research

When heated, metal hydroxides decompose to produce a metal oxide and water. Selected the correct balanced - brainly.com









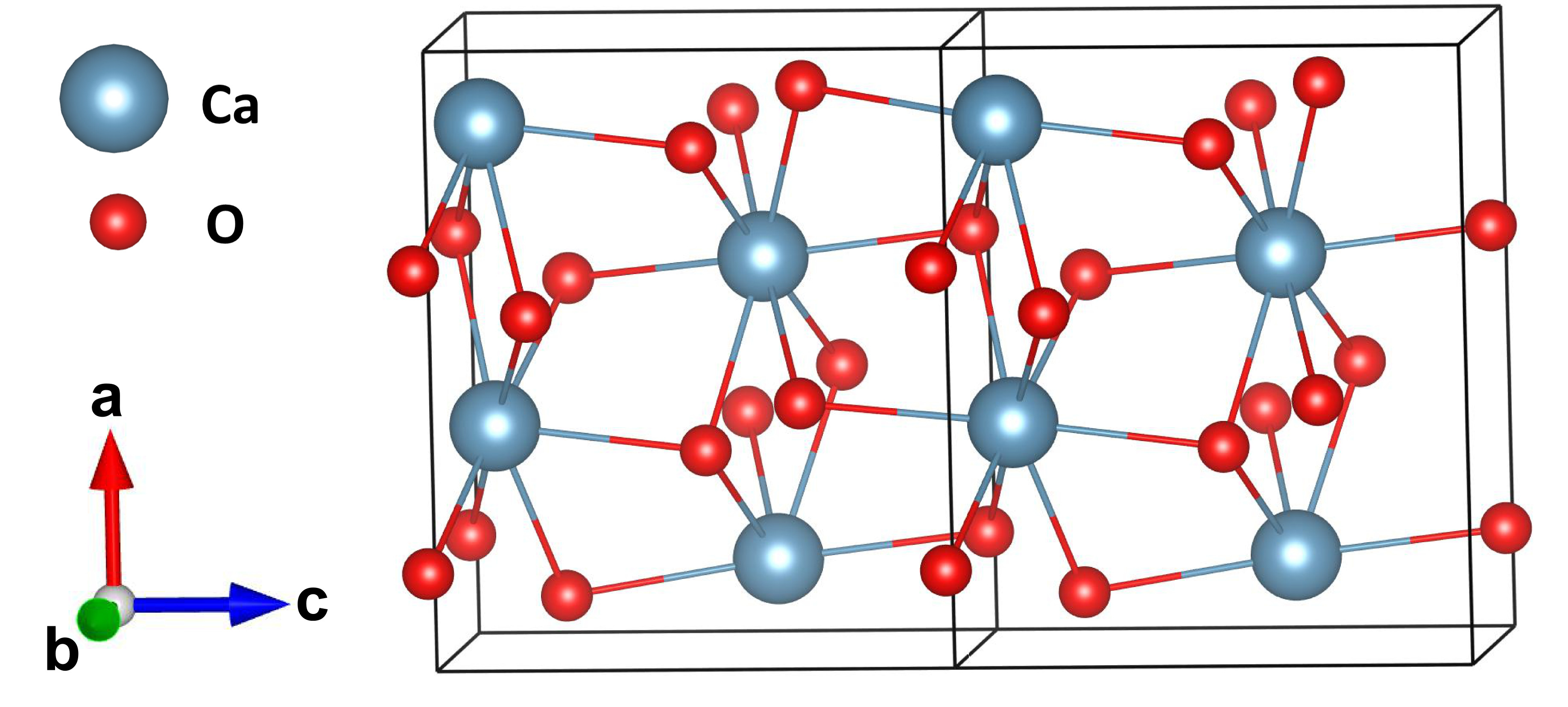


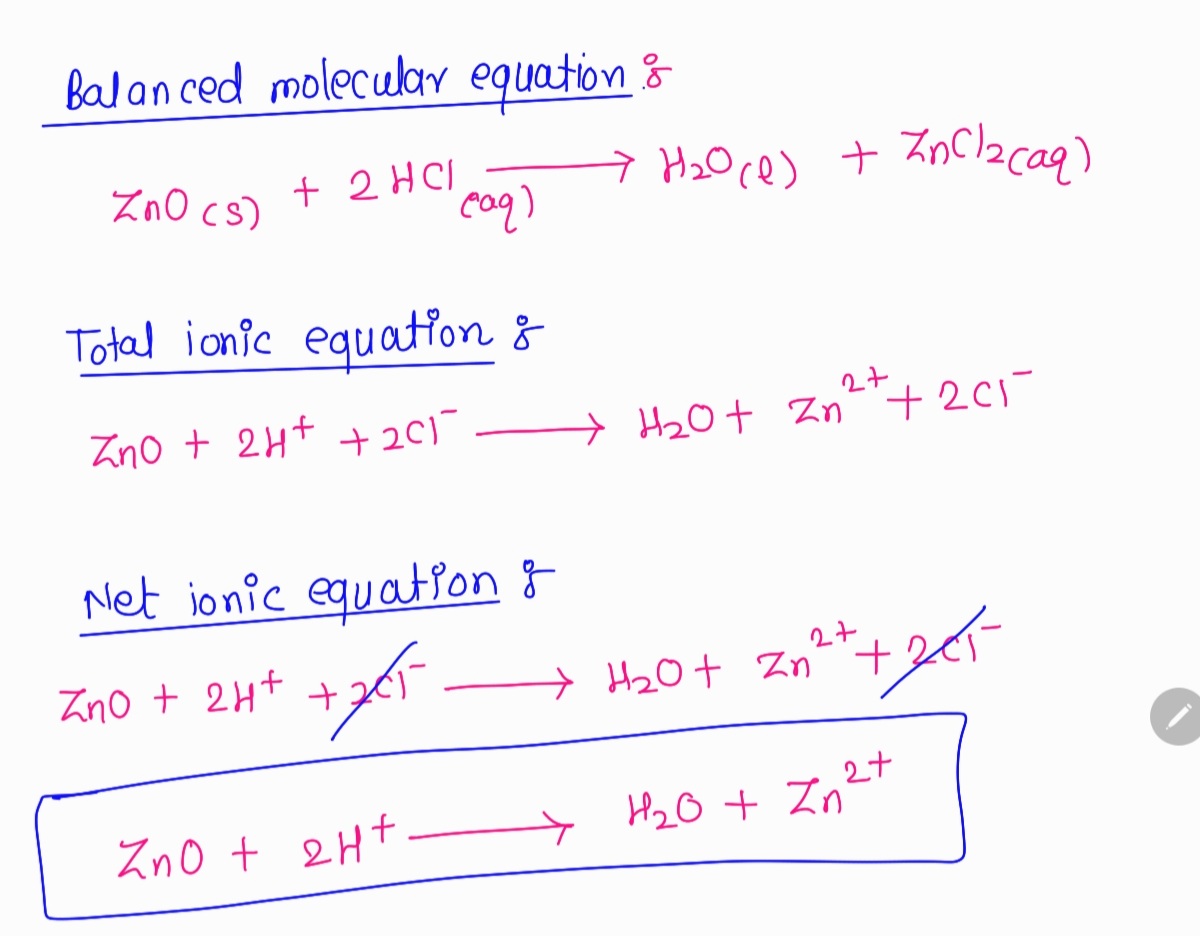
![Punjabi] What type of reactions are represented by following equation Punjabi] What type of reactions are represented by following equation](https://static.doubtnut.com/ss/web-overlay-thumb/10335489.webp)