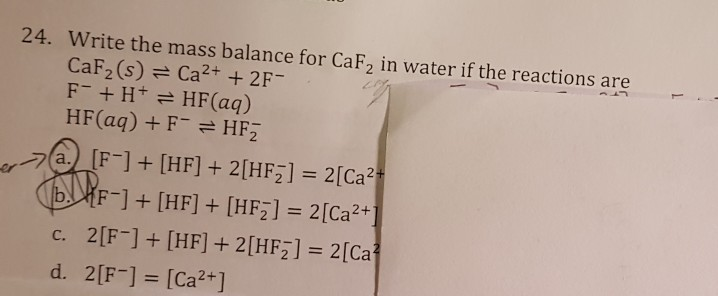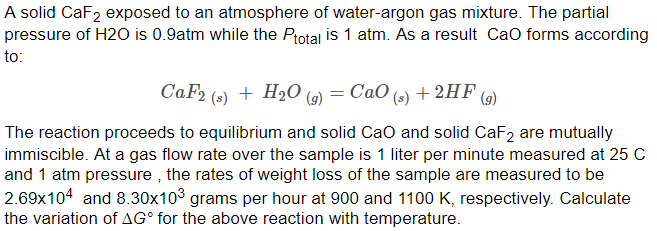Luminescence of Eu3+ Activated CaF2 and SrF2 Nanoparticles: Effect of the Particle Size and Codoping with Alkaline Ions | Crystal Growth & Design

Charge Reversal Behavior at the CaF2/H2O/SDS Interface as Studied by Vibrational Sum Frequency Spectroscopy | The Journal of Physical Chemistry B

CaF2 97 %. Le spath fluor Poudre humide/ CaF2 en poudre, de spath fluor Poudre humide - Chine Fluoraper, le fluorure de calcium

Solubility Data for the CaF 2 + MnSO 4 + H 2 O Ternary System at 298.15... | Download Scientific Diagram

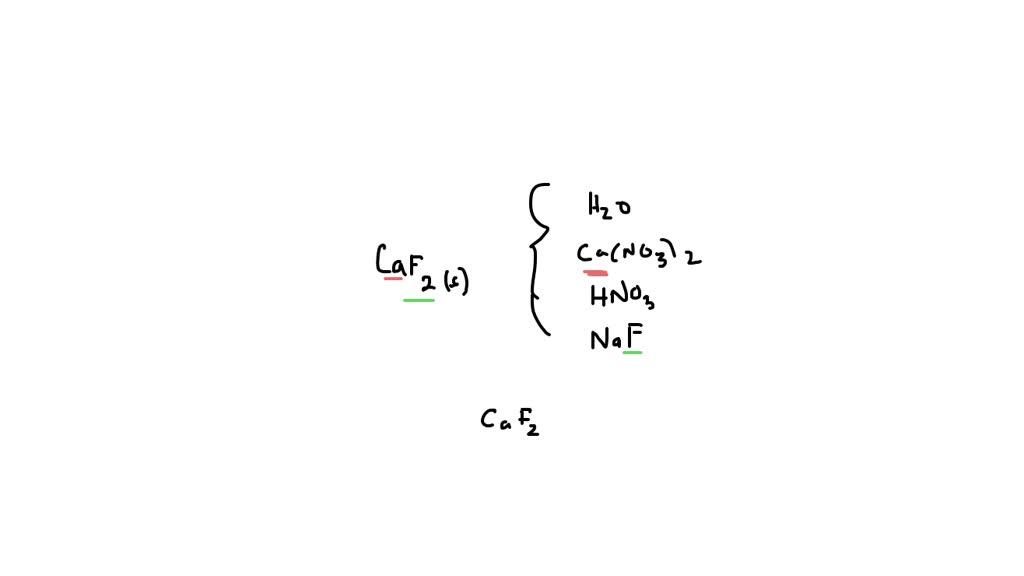
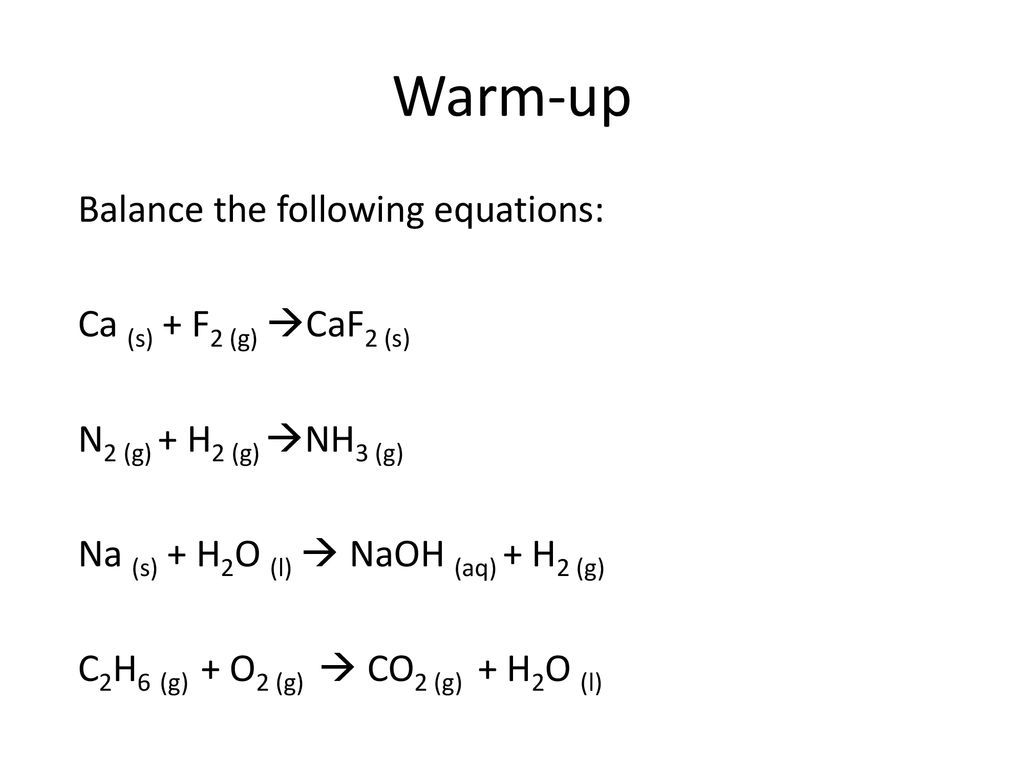
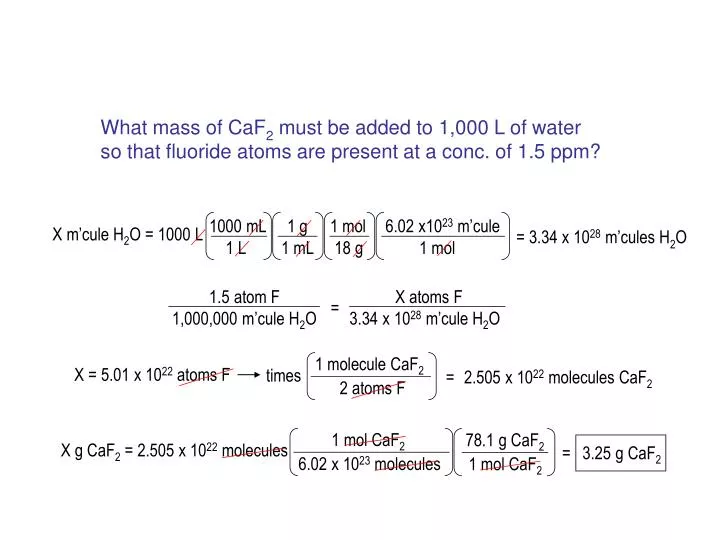
SOLVED: Which of the following will increase the solubility of calcium fluoride in water? CaF2 (s) = Ca2+ (aq) + 2 F- (aq) Adding calcium nitrate Adding a strong base Adding sodium





![Solved [2] (a) Consider the reaction: | Chegg.com Solved [2] (a) Consider the reaction: | Chegg.com](https://media.cheggcdn.com/study/0cd/0cd25e73-2e52-4764-8524-5f8d96b0fc58/image)