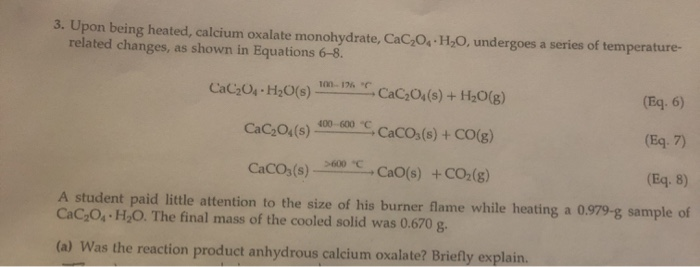Calcium oxalate, CaC2O4.H2O, is a sparingly soluble salt of analytical and physiological importance. - Sarthaks eConnect | Largest Online Education Community
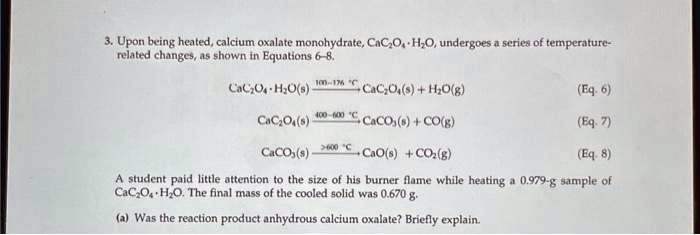
SOLVED: 3. Upon being heated, calcium oxalate monohydrate, CaC2O4 H2O, undergoes a series of temperature-related changes, as shown in Equations 6-8. CaC2O4 H2O(s) -> CaC2O4(s) + H2O(g) (Eq. 6) CaC2O4(s) + H2O(g) ->

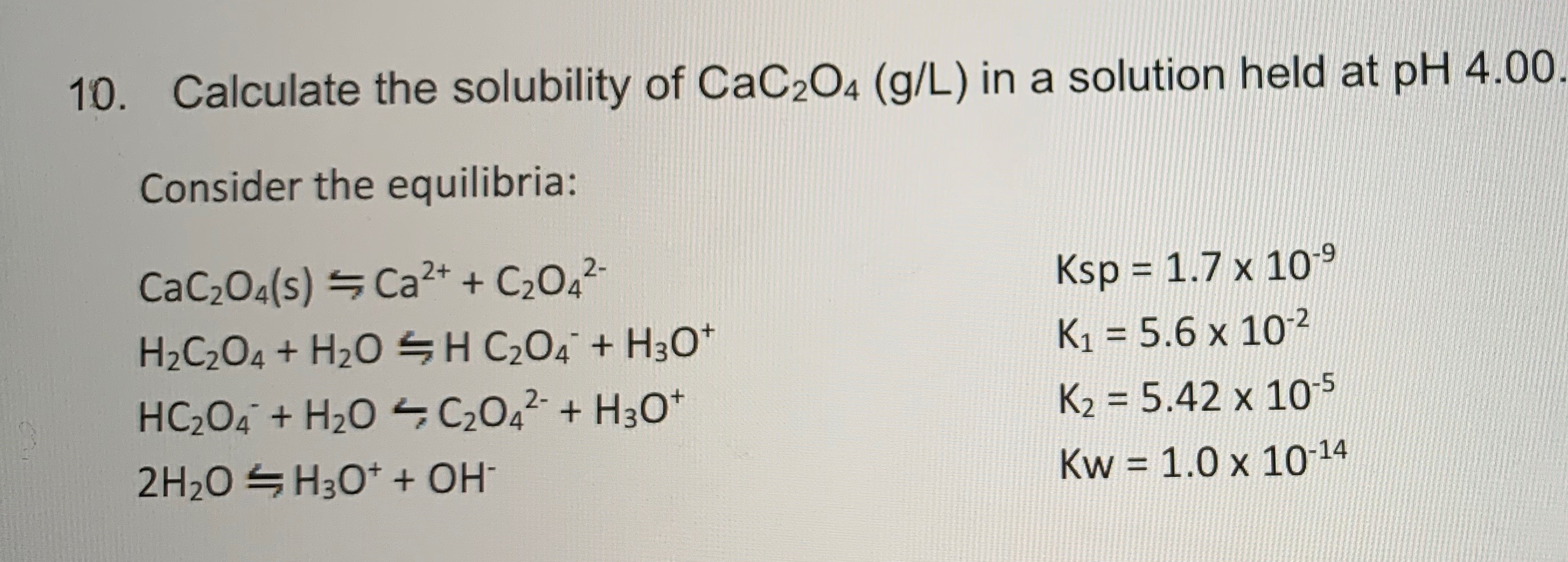
How many grams of CaC2O4 will dissolve in distilled water to one litre saturated solution? solubility product of CaC2O4 is 2.5 x 10-9 mol? L-2 and its molecular weight is 128.
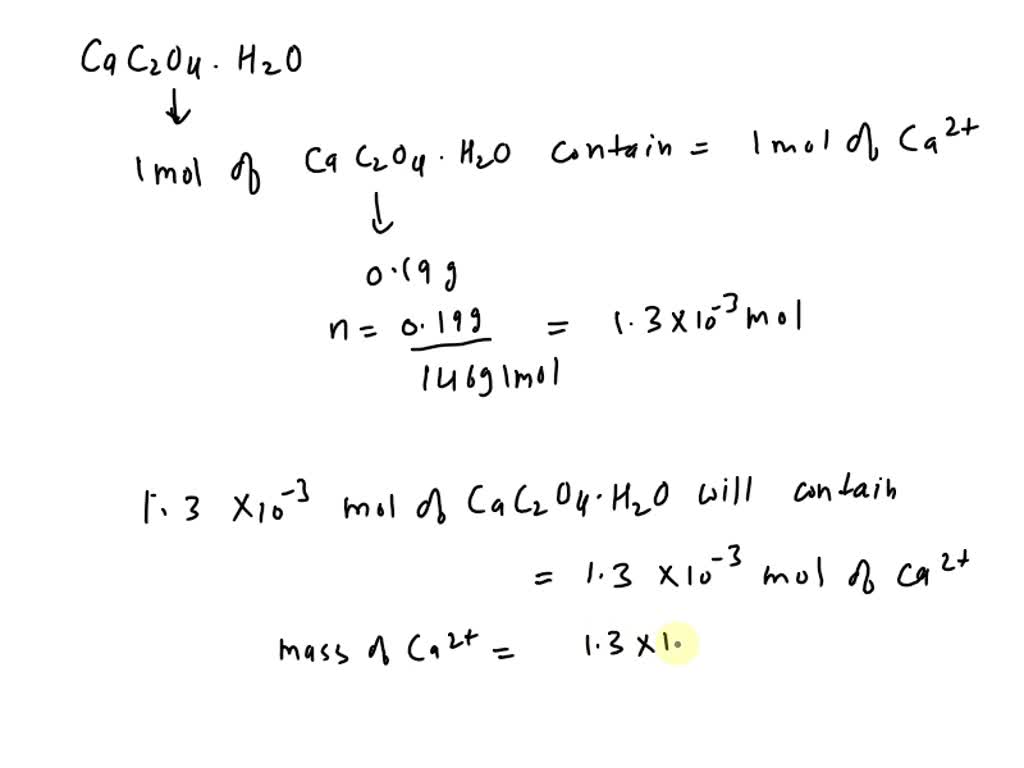
SOLVED: What is the concentration of calcium (Ca2+) in calcium oxalate monohydrate (CaC2O4·H2O)? Ca2+(aq) + C2O42-(aq) + H2O(l) â†' CaC2O4·H2O(s) The mass of calcium oxalate monohydrate is 0.190 grams. What is the [

SOLVED: Gravimetric Determination of Calcium as CaC2O4 H2O Introduction Calcium ion can be analyzed by precipitation with oxalate basic solution to form CaC2O4 H2O. The precipitate is soluble in acidic solution because

Thermal Decomposition of CaC2O4·H2O Studied by Thermo-Raman Spectroscopy with TGA/DTA | Analytical Chemistry

SOLVED: Upon being heated, calcium oxalate monohydrate (CaC2O4 * H2O) undergoes a series of temperature-related changes, as shown in Equations 6-8. CaC2O4 * H2O(s) â†' CaC2O4(s) + H2O(g) (Eq: 6) CaC2O4(s) â†'

Thermal Decomposition of CaC2O4·H2O Studied by Thermo-Raman Spectroscopy with TGA/DTA | Analytical Chemistry
MSE310 Lecture 2 Thermogravimetric Analysis (TGA) The TGA technique measures the mass of a sample as it is heated, cooled or he

Influence of residence time on conversion ratio of calcium oxalate (a)... | Download Scientific Diagram

Thermal Decomposition of CaC2O4·H2O Studied by Thermo-Raman Spectroscopy with TGA/DTA | Analytical Chemistry