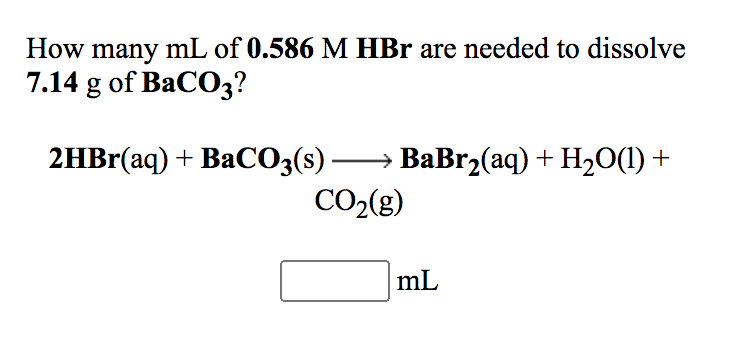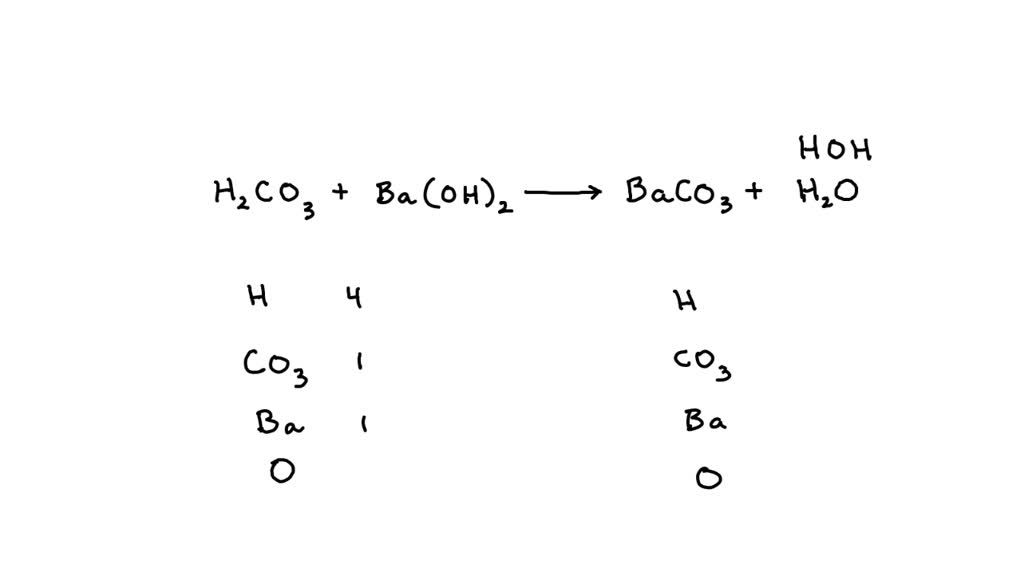a. SO2 + ? → H2SO3 b. ? +H2O → KOH c. ? + ?→ CaCO3 d. CO2 + ? → BaCO3 + H2O e. ? + H2S04 → MgSO4 + H2O f. Fe + HCl– → ? + ? g. Al203 + H2SO4 → ? + H2O h. N

Question Video: Determining the Products of the Neutralization Reaction of Barium Hydroxide Ba(OH)₂ with Carbonic Acid H₂CO₃ | Nagwa
Trajectory and timescale of oxygen and clumped isotope equilibration in the dissolved carbonate system under normal and enzymati

Vidéo de question : Identifier la substance qui contient à la fois des liaisons ioniques et des liaisons covalentes | Nagwa

Date twe of bor WYSICAL SUENCE Works Balance the following equations: 1. AL + N2 - AIN 2. Fe + 02 - Fe3O4 Caco - CaO + CO2 NH.NO, N2O + H2O

PPT - What is the difference between a chemical reaction and physical change? PowerPoint Presentation - ID:5813241
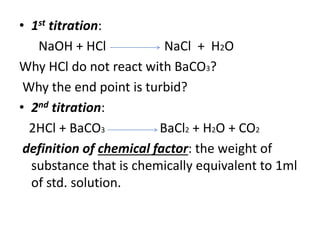
Solved: The chemical equation here describes a reaction between barium carbonate and nitric acid. [algebra]

Signaling Modulation via Minimal C-Terminal Modifications of Apelin-13 | ACS Pharmacology & Translational Science

Synthetic pathway of 1. a) NH2OH·HCl, BaCO3, Pd/C, N2H4·H2O, reflux in... | Download Scientific Diagram